
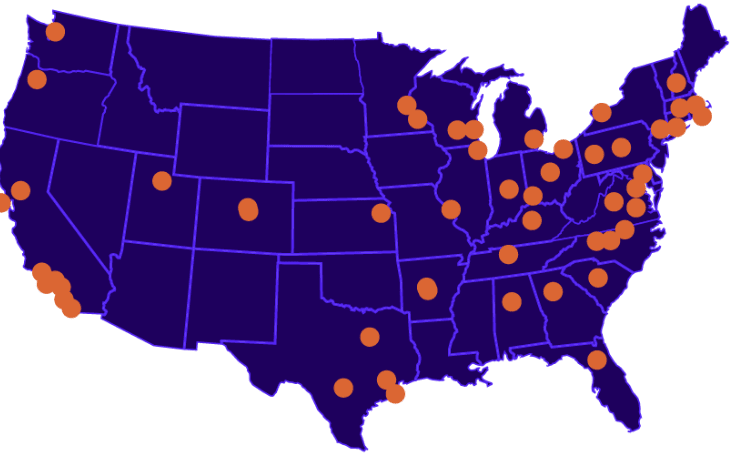
The Largest Federated Network for Regulatory-Compliant EHR-Based Research
Stay Connected with the ENACT Network
Subscribe to our email newsletter for exclusive updates on network developments, new tools and resources, interactive multimedia content, and more. Whether you’re a researcher, clinician, or collaborator, our newsletter keeps you informed and inspired.
Sign up to stay in the loop.
ENACT enables…
Regulatory-Compliant EHR-Based Research
Improved data quality
Access to trainees for educational purposes
AI and machine learning analysis to large-scale EHR data sets
Informatics experts to develop and validate new tools
Clinical Decision Support
Current Capabilities of ENACT
The ENACT Network allows researchers to explore and validate feasibility for clinical studies from their desktops, providing a real-time platform for this purpose within the CTSA consortium.
It is equipped to assist researchers in designing and completing clinical studies, ensuring that the data and processes are secure and compliant with relevant regulations such as HIPAA.
Researchers can use the network to query patient data from participating sites, which includes demographics, diagnoses, lab results, and medications prescribed. This is to help determine the availability of suitable candidates for clinical studies.

ENACT History/Overview
ACT Ontology Updates: The network utilizes the ACT Ontology, which in version 4.1 includes OMOP support, a new research ontology branch, and new vaccination and zip code ontologies.
SHRINE Upgrade: The Shared Health Research Information Network (SHRINE) is currently version 3.3.2, which brought enhanced features such as updated branding and a direct link to the ENACT publication policy.
i2b2 Versions: The network has incorporated the i2b2 software for data management and research facilitation. Note that the i2b2 software is compatible with OMOP and i2b2 databases. The network is currently on version 1.8.1a.
These software elements reflect the ENACT Network’s commitment to maintaining a robust, up-to-date technical infrastructure to support clinical research and data analysis. New software and ontology upgrades will come in the late summer of 2025.
